Research interests revolve around areas of scientific and societal importance such as energy and environment related issues. Examples includes solvation of glucose and dioxin in ionic liquids (ILs), solvation of metal organic framework (MOFs) in ILs for capturing CO2 and modeling energy storage materials such as improving ion transport phenomena in lithium batteries, modeling of polymer electrolytes composed of ILs for lithium batteries and understanding of microscopic properties such as dielectric relaxation and ionic conductivity of ILs mixture. Different simulation methods like classical molecular dynamics (MD), ab initio MD and quantum mechanics are employed for modeling of interesting systems with applications in the field of biology, energy and materials by bridging theory and experiment. We attempt to mimic actual experimental conditions in our simulations, facilitating a direct comparison of simulation results with experimental. In this way, we will be able to provide microscopic detail to explain macroscopic phenomena.
Dielectric relaxation and ion conductivity of polymerized imidazolium based ionic liquids and
their mixtures: Microwave and Far-IR Properties
style="text-align:justify">Ionic liquids (ILs) consisting of bulky organic cations paired with organic or inorganic anions are characterized by anisotropy in the charge distribution and shape. These attributes make ILs an interesting and challenging class of materials to understand their dielectric and related properties, as they have characteristics of both polar liquids and ionic melts.
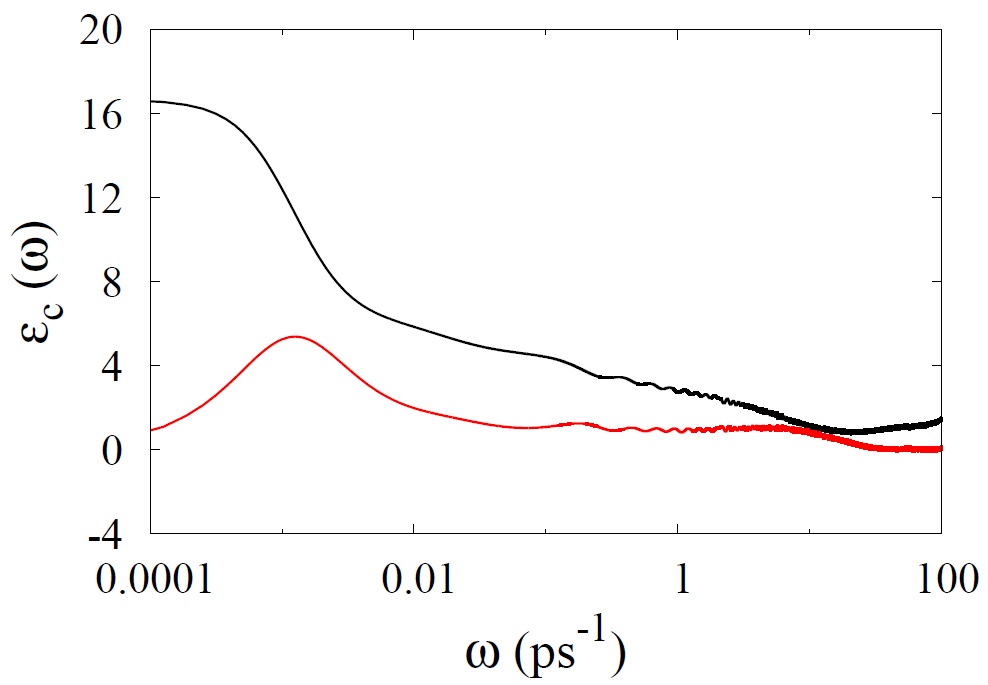
The dielectric relaxation and ion conductivity is a very powerful tool for understanding the molecular-level dynamics in ion-containing liquids such as ILs. Dielectric relaxation probes polarization as the response of the sample to a time-dependent electric field giving a valuable insight into molecular mobility of ILs. In ILs, a polarization essentially originates from the orientational fluctuations of permanent dipoles of cation or anion and from ion translational motions. Typical dielectric spectra is shown in Figure. The attractive interactions between cations and anions are dominated by electrostatic forces and the size of ions. It would be interesting to analyze the dielectric relaxation and ion conductivity of polymerized imidazolium based ILs with different chain length and their mixture imidazolium based ILs.
Analysis of molecular interactions such as hydrogen bonding and their consequences to spectrum in Mid IR, Far-IR and terahertz region
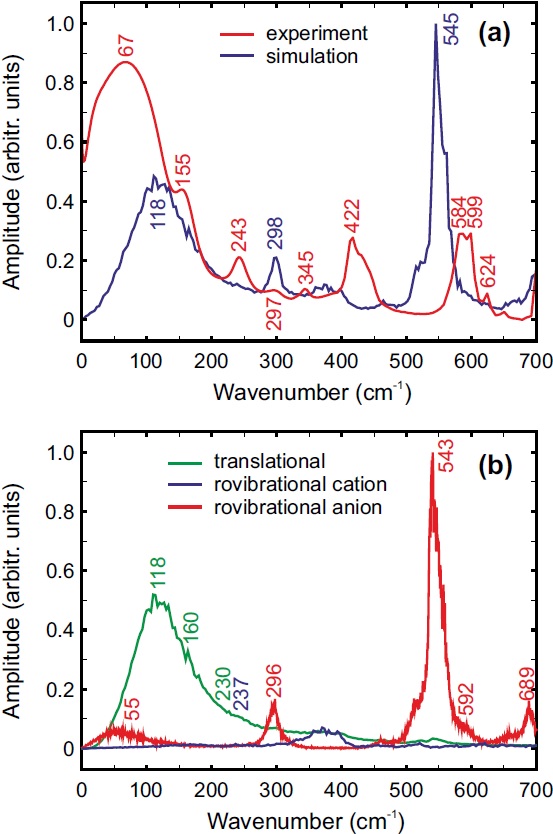
The far-IR (FIR) and terahertz spectroscopy thus offers an excellent experimental tool to probe individual and collective (inter)molecular motions subject to the influence of local structure. As such, dielectric absorption data have been very useful in theoretical modeling and understanding of dynamics in polar solvents and ionic solutions.
In principle, the hydrogen bonded networks can be studied by measuring the low frequency range below 400 cm-1 using the far IR spectroscopy. However, very few infrared studies of ILs have been performed in the far infrared regime. Experimental difficulties arise from the very low intensities of infrared sources. Herein, simulation results play an important role in the assignment of the vibrations. In would be interesting to analyze the effect of different anions and the different chain length of cation on the intermolecular vibrational band. The simulation results will be comparing with the available experimental low frequency spectrum.
Modelling of polymer electrolytes integrated with ILs for lithium batteries
The stringent demand for portable power sources characterized by high energy and/or high power densities, good recyclability, reliability, and safety continues to generate a great deal of research interest from both academia and industry world-wide. Unfortunately, the thermal instability of the alkyl carbonates which are the most common electrolytes for the Li-ion batteries tends to restrict the utility of such batteries at elevated temperatures. Furthermore, high volatility and flammability of the conventional organic electrolytes pose a serious safety issue for their use in the consumer and transportation markets. To address such issues, a significant effort has been focused on the synthesis and utilization of nonflammable electrolytes, such as ionic liquids, gel-polymer matrices, and small molecule additives.
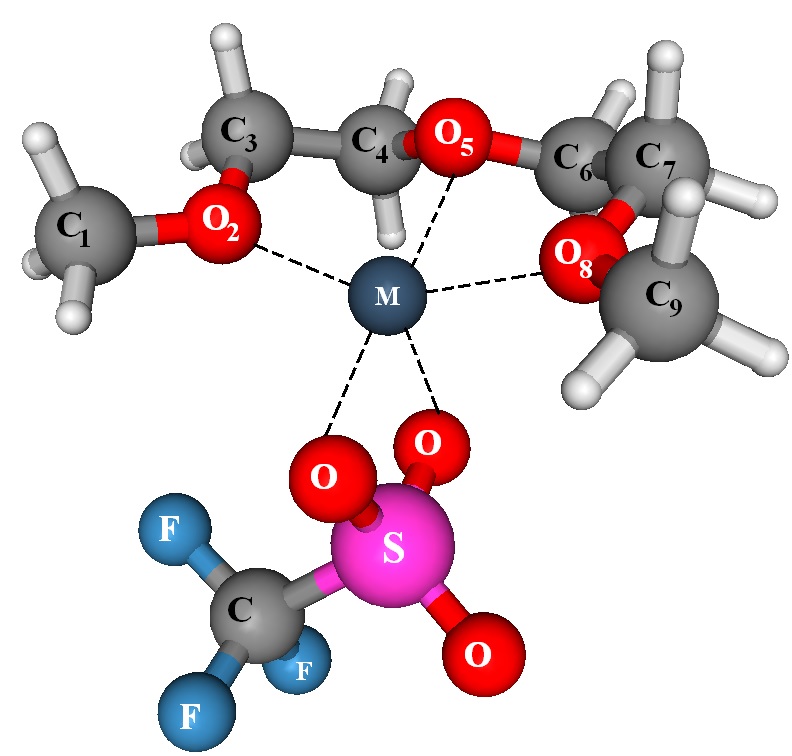
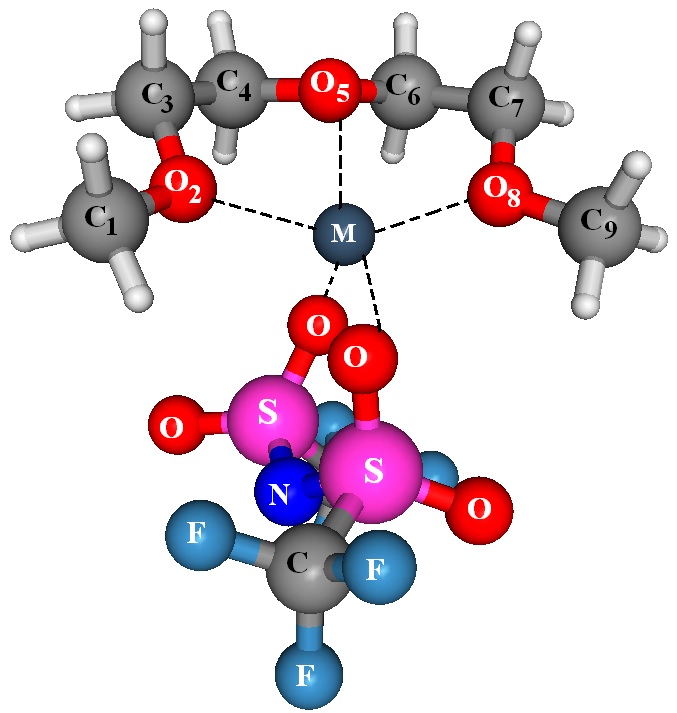

The prototypical solid polymer electrolyte (SPE) is PEO-LiX, prepared by blending PEO with lithium salt (LiX) of a large anion. A variety of ionic salts, such as LiCF3SO3 or Li(CF3SO2)2N dissolve in PEO to form SPE, exhibit high ionic conductivity, which facilitate their use in fuel cells, secondary batteries and other electrochemical devices. The transport of Li+ ions in these electrolytes has been associated with the local relaxation and segmental motion of the amorphous regions in the PEO chains. These polymers often show higher crystallinity at lower temperatures and the electrolytes exhibit low room temperature ionic conductivity (typically = 10- 5 S/cm). This necessitates operation at higher temperatures (generally, > 70 °C) for successful utilization of them in practical applications.
Another approach recently adopted has been to incorporate ILs in PEO-LiX SPE. ILs are molten salts having a low melting point and exist as liquids at or below room temperature. Unlike molecular solvents, ILs are nonflammable and nonvolatile and hence cause no detrimental effect on the safety aspects of the battery. Different types of ILs such as those with cations based on imidazolium , pyrrolidinium, piperidinium , morpholinium and quarternary ammonium have been investigated as electrolytes for battery/capacitor applications either solely and or as the solvent when incorporated in gel/polymer electrolytes.